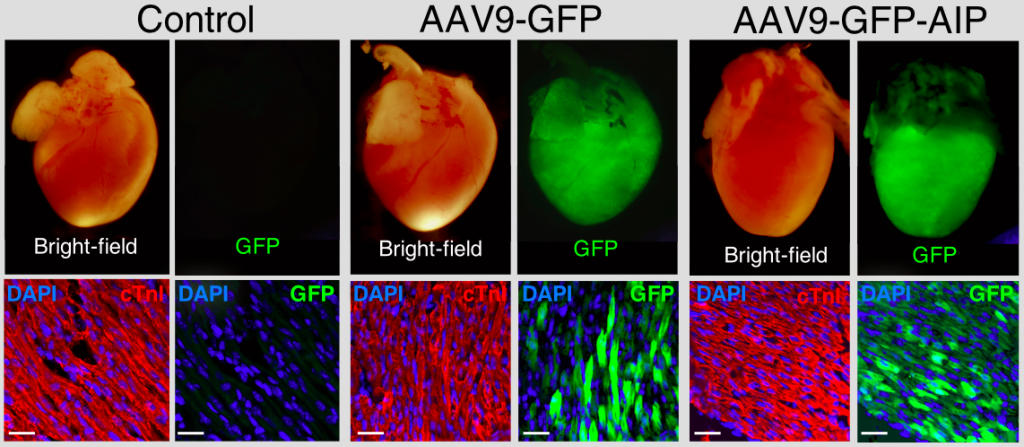
Cardiac Gene Therapy– Patients with inherited cardiovascular disorders including inherited arrhythmia syndromes are at life-long risk for arrhythmias, heart failure and even sudden death. To develop new therapies for these patients, we used induced pluripotent stem cells (iPSCs) derived from patients with catecholaminergic polymorphic ventricular tachycardia (CPVT) to model the syndrome in the laboratory. Patients with CPVT have life-threatening arrhythmias during times of stress or exercise and current therapies are inadequate to fully protect patients. Dominant mutations in the ryanodine receptor 2 (RYR2), an intracellular calcium release channel, are responsible for the majority of CPVT cases and leads to excessive calcium leak during beta-adrenergic stimulation of cardiomyocytes (CMs). We identified that abnormal activation of calcium/calmodulin dependent kinase II (CaMKII) effectively unmasks the arrhythmogenic phenotype of differentiated cardiomyocytes from CPVT patients by phosphorylation at a specific RYR2 residue. Therefore, suppression of abnormal activation of CaMKII in the heart could be a viable therapy for patients with CPVT and other cardiac disorders related to CaMKII dysfunction. Unfortunately, normal functioning of CaMKII within the CNS is critical for learning and memory. To overcome this limitation, we targeted expression of a potent CaMKII peptide inhibitor just to the heart through delivery of engineered adeno-associated virus type 9 (AAV9) viral particles. This treatment successfully suppressed arrhythmia in two mouse models of CPVT strongly suggesting a pathway towards a viable gene therapy. We are now refining this process towards a first in human clinical trial and further expanding it to treat other forms of inherited cardiac disease.
Improving Stem Cell Models of Cardiac Disease– Induced pluripotent stem cells (iPSCs) have revolutionized disease modeling and promise to usher in a new era of personalized medicine. However, several challenges remain to make this revolutionary technology widely available and to reliably improve clinical management and outcome prediction. One current limitation is that iPSC derived cardiomyocytes (iPSC-CMs) are functionally immature as compared to adult cells. This immaturity may limit their potential to accurately reflect clinical phenotypes. Therefore, in collaboration with Dr. Richard Lee at Harvard University we assisted in the characterization of a novel method to improve the maturity of iPSC-CMs through treatment with the mTOR inhibitor Torin1. We are currently characterizing the electrical changes induced by Torin1 to improved modeling of inherited arrhythmia syndromes. In addition to being functionally immature, iPSC-CMs are also a mixture of different cardiac cell types including ventricular, atrial and nodal cells. To improve chamber specificity, we are combining, cell-type specific differentiation, molecular beacons and optogenetics to make effective atrial and ventricular engineered heart tissues for arrhythmia modeling.
Novel Mechanisms of Arrhythmia– We recently identified a large family with severe long-qt syndrome as well as developmental disease and other extra-cardiac abnormalities. Genetic testing revealed a novel mutation in a non-ion channel related protein. We are using both iPSC and animal models to characterize this novel form of inherited arrhythmia syndrome and its implications for cardiac physiology and disease.